Multiple Choice
Write the equilibrium equation for the reverse reaction: 4 CH4 (g) + 6 O2 (g) ⇌ 4 CO (g) + 8 H2O (g)
A) Kc' = 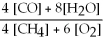
B) Kc' = 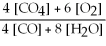
C) Kc' = 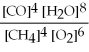
D) Kc' = 
Correct Answer:

Verified
Correct Answer:
Verified
Q22: The reaction CaCO<sub>3</sub>(s)⇌ CaO(s)+ O<sub>2</sub>(g)is endothermic 298
Q23: Given the reaction: 2 HI ⇌ H<sub>2</sub>
Q24: The reaction A<sub>2</sub> + B<sub>2 </sub>⇌ 2AB
Q25: For the reaction shown below,which change in
Q26: Ammonium carbamate can dissociate into gases at
Q28: For the reaction,A(g)+ 2 B(g)⇌ 2 C(g),K<sub>c</sub>
Q29: Given the reaction at a certain temperature:
Q30: Cyclohexane,C<sub>6</sub>H<sub>12</sub>,undergoes a molecular rearrangement in the presence
Q31: Cyclohexane (C<sub>6</sub>H<sub>12</sub>)undergoes a molecular rearrangement in the
Q55: For the isomerization reaction: butane ⇌ isobutane<br>K<sub>p</sub>