Multiple Choice
What is the approximate pH of a solution X that gives the following responses with the indicators shown? 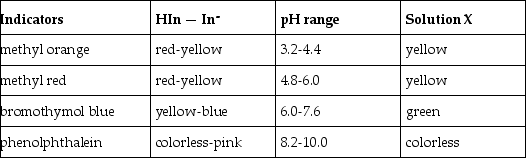
A) 3.2 - 4.4
B) 4.8 - 6.0
C) 6.0 - 7.6
D) 8.2 - 10.0
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q38: Calculate the pH of a 0.100 M
Q95: Undersea flora prefer a maximum concentration of
Q96: A 0.050 M solution of hydroxylamine,NH<sub>2</sub>OH,having K<sub>b</sub>
Q97: Erythromycin is a basic antimicrobial with pK<sub>b</sub>
Q98: Determine the acid dissociation constant for a
Q99: Calculate the pH for an aqueous solution
Q101: Benzoic acid (C<sub>6</sub>H<sub>5</sub>CO<sub>2</sub>H = HBz)solutions are sometimes
Q103: An Arrhenius base is best defined as
Q104: Using the conjugate acid-base pairs listed below,complete
Q105: Indicate all the Br∅nsted-Lowry acids in the