Multiple Choice
What is the approximate pH of a solution X that gives the following responses with the indicators shown? 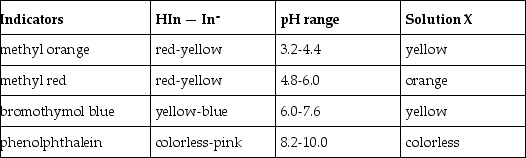
A) 3.2 - 4.4
B) 4.8 - 6.0
C) 6.0 - 7.6
D) 8.2 - 10.0
Correct Answer:

Verified
Correct Answer:
Verified
Q168: A Br∅nsted-Lowry acid is best defined as
Q169: Which of the following can be classified
Q170: An acidic solution at 25°C has<br>A)[H<sub>3</sub>O<sup>+</sup>] >
Q171: Methylamine CH<sub>3</sub>NH<sub>2</sub>,has a base dissociation constant of
Q172: Which one of the following is least
Q174: For Cu<sup>2+</sup> and CO<sub>2</sub>,which will behave as
Q175: The value of K<sub>a</sub> for a 0.250
Q176: Which one of the following species acts
Q177: At 50°C the value of K<sub>w</sub> is
Q178: SO<sub>3</sub> reacts with H<sub>2</sub>O to form H<sub>2</sub>SO<sub>4</sub>.Which