Multiple Choice
What is the approximate pH of a solution X that gives the following responses with the indicators shown? 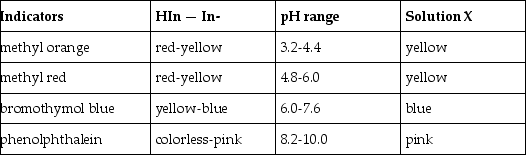
A) 4.8 - 6.0
B) 6.0 - 7.6
C) 7.6 - 8.2
D) > 8.2
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: Calculate the pH of a 0.020 M
Q2: BF<sub>3</sub> and NH<sub>3</sub> undergo a Lewis acid-base
Q3: Which Br∅nsted-Lowry base has the strongest conjugate
Q5: A solution with a hydrogen ion concentration
Q6: In the aquation reaction Co<sup>2+</sup> + 6
Q7: Which of the following should have the
Q8: What are the Br∅nsted-Lowry acids in the
Q9: What is the pH of a solution
Q10: What is the hydronium ion concentration of
Q11: Which of the following is a weak