Multiple Choice
In the following reaction the unshaded spheres represent H atoms. 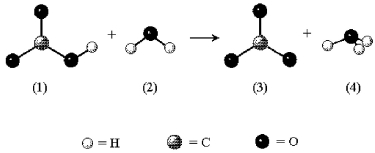
-Identify the Br∅nsted-Lowry acid/base conjugate pairs.
A) (1) /(2) and (3) /(4)
B) (1) /(3) and (2) /(4)
C) (1) /(4) and (2) /(3)
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q187: What is the strongest acid among the
Q188: Calculate the hydronium ion concentration in an
Q189: Which Br∅nsted-Lowry acid has the strongest conjugate
Q190: What is the conjugate acid of the
Q191: What is the pH of a 0.20
Q193: The equilibrium constant for the reaction below
Q194: Normal rainfall has a concentration of OH<sup>-</sup>
Q195: A solution with a hydroxide ion concentration
Q196: In the following reaction the unshaded spheres
Q197: Acetic acid CH<sub>3</sub>COOH,has an acid dissociation constant