Multiple Choice
The following pictures represent aqueous solutions of three acids HA (A = X,Y,or Z) ;water molecules have been omitted for clarity. 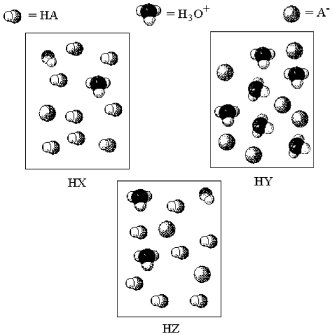
-Which acid,if any,is a strong acid?
A) All are strong acids.
B) HX and HZ
C) HY
D) None are strong acids.
Correct Answer:

Verified
Correct Answer:
Verified
Q53: In order for the reaction A<sup>-</sup> +
Q54: The following pictures represent equal volumes of
Q55: The pH of a 0.055 M KOH
Q56: What is the pH of a 2.4
Q57: An acidic solution at 25°C will have
Q59: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -Of the elements
Q60: Which of the following salts are acidic?<br>A)LiCl,NaCl,KCl<br>B)NH<sub>4</sub>Cl,CuCl<sub>2</sub>,AlCl<sub>3</sub><br>C)NaCH<sub>3</sub>CO<sub>2</sub>,LiCH<sub>3</sub>CO<sub>2</sub>,RbCH<sub>3</sub>CO<sub>2</sub><br>D)KCl,NH<sub>4</sub>Cl,Na<sub>2</sub>CO<sub>3</sub>
Q61: What is the strongest monoprotic acid of
Q62: Phenobarbital has a pK<sub>a</sub> = 7.4.Compared to
Q188: Calculate the hydronium ion concentration in an