Multiple Choice
The following pictures represent aqueous solutions of three acids HA (A = X,Y,or Z) ;water molecules have been omitted for clarity. 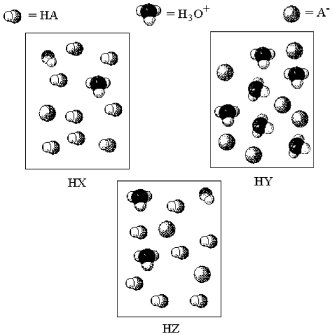
-Which acid has the smallest value of Ka?
A) HX
B) HY
C) HZ
D) All have the same Ka value.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q111: Calculate the hydroxide ion concentration in an
Q112: If an equal number of moles of
Q113: What is the hydronium ion concentration of
Q114: Calculate the pH for an aqueous solution
Q115: What is the pH of a solution
Q117: Potassium hydrogen phthalate (molar mass = 204.2
Q118: The following pictures represent aqueous solutions of
Q119: If you know K<sub>b</sub> for ammonia,NH<sub>3</sub>,you can
Q120: Potassium hydrogen phthalate (molar mass = 204.2
Q121: What statement is most consistent for an