Multiple Choice
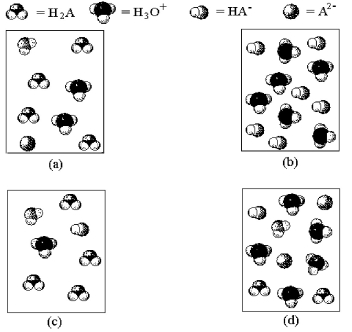
-Which of the above pictures represents a solution of a weak diprotic acid H2A for which Ka1 >> Ka2? (Water molecules have been omitted for clarity. )
A) picture (a)
B) picture (b)
C) picture (c)
D) picture (d)
Correct Answer:

Verified
Correct Answer:
Verified
Q86: Calculate the pH of a 0.20 M
Q121: What statement is most consistent for an
Q122: Vinegar is a 5.0% solution by weight
Q124: Which of the following Br∅nsted-Lowry acids does
Q125: The following pictures represent equal volumes of
Q127: What is the relationship between K<sub>a</sub> and
Q128: Para-Aminobenzoic acid (PABA),p-H<sub>2</sub>NC<sub>6</sub>H<sub>4</sub>(COOH),is used in some sunscreens
Q129: What is the second stepwise equilibrium constant
Q131: What is the pH of a solution
Q196: In the following reaction the unshaded spheres