Multiple Choice
The following pictures represent equal volumes of aqueous solutions of three acids HA (A = X,Y,or Z) ;water molecules have been omitted for clarity. 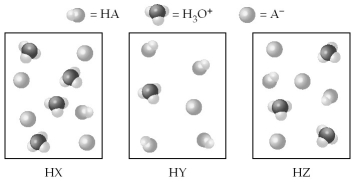
-Arrange the acids in order of increasing acid strength.
A) HZ < HY < HX
B) HY < HZ < HX
C) HZ < HX < HY
D) HX < HZ < HY
Correct Answer:

Verified
Correct Answer:
Verified
Q137: What is the hydronium ion concentration and
Q138: What is the weakest acid among the
Q139: Calculate the pH for an aqueous solution
Q140: The following pictures represent solutions of three
Q141: Which one of the following salts,when dissolved
Q143: What is the pH of a solution
Q144: What is the pH of a 0.040
Q145: Calculate the concentration of bicarbonate ion,HCO<sub>3</sub><sup>-</sup>,in a
Q146: A 1.25 × 10<sup>-4</sup> M solution of
Q147: A weak base ionizes in water as