Multiple Choice
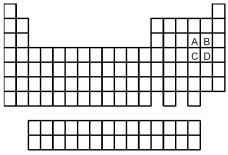
-Of the elements indicated on the periodic table shown above,which forms the strongest binary acid,H2X or HX,where 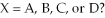
A) A
B) B
C) C
D) D
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q106: When dissolved in water,which compound is generally
Q197: Acetic acid CH<sub>3</sub>COOH,has an acid dissociation constant
Q198: What is the pH of a solution
Q199: A solution with hydronium ion concentration [H<sup>+</sup>]
Q200: From the following chemical reactions determine the
Q201: The following pictures represent equal volumes of
Q203: Which is not a hydrate of a
Q205: In the following reaction the unshaded spheres
Q206: Arrange the following 0.10 M aqueous solutions
Q207: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -Of the elements