Multiple Choice
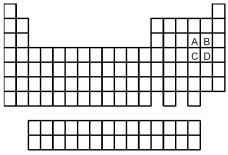
-Of the elements indicated on the periodic table shown above,which forms the weakest oxoacid acid with the formula H2XO3 or HXO3,where X = A,B,C,or D?
A) A
B) B
C) C
D) D
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q162: What is the strongest acid among the
Q163: What is the pH of a 0.300
Q164: In order for the reaction HA +
Q165: Which one of the following salts,when dissolved
Q166: What is the strongest acid among the
Q168: A Br∅nsted-Lowry acid is best defined as
Q169: Which of the following can be classified
Q170: An acidic solution at 25°C has<br>A)[H<sub>3</sub>O<sup>+</sup>] >
Q171: Methylamine CH<sub>3</sub>NH<sub>2</sub>,has a base dissociation constant of
Q172: Which one of the following is least