Multiple Choice
CO2 reacts with H2O to form HCO3- and H+.Which picture below correctly represents the curved arrow notation for the initial Lewis acid-Lewis base interaction in this reaction;what is the Lewis acid and the Lewis base? 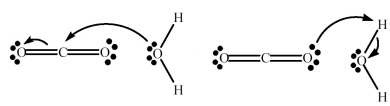 (1) (2)
(1) (2)
A) Picture (1) is correct;H2O is the Lewis acid and CO2 is the Lewis base.
B) Picture (1) is correct;CO2 is the Lewis acid and H2O is the Lewis base.
C) Picture (2) is correct;H2O is the Lewis acid and CO2 is the Lewis base.
D) Picture (2) is correct;CO2 is the Lewis acid and H2O is the Lewis base.
Correct Answer:

Verified
Correct Answer:
Verified
Q80: Identify the Lewis acid that acts as
Q81: What is the pH of a solution
Q82: At 50°C the value of K<sub>w</sub> is
Q83: The following pictures represent aqueous solutions of
Q84: A 0.50 M KNO<sub>2</sub> solution will have
Q86: Calculate the pH of a 0.20 M
Q87: What is the pH of a 0.020
Q88: What is the pH of a 0.30
Q89: The acid strength of an oxoacid having
Q90: What is the hydroxide ion concentration of