Multiple Choice
What is the hydronium ion concentration and the pH for an aqueous solution of NH3 that has a hydroxide ion concentration of 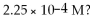
A) 4.44 × 10-10 M and 4.65
B) 4.44 × 10-10 M and 9.35
C) 4.44 × 10-11 M and 3.65
D) 4.44 × 10-11 M and 10.35
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q180: Identify the Lewis acid that acts as
Q181: What is the strongest acid of the
Q182: What is the strongest acid among the
Q183: What is the hydronium ion concentration of
Q184: Calculate the concentration of bicarbonate ion,HCO<sub>3</sub><sup>-</sup>,in a
Q186: What is the pH of a 0.020
Q187: What is the strongest acid among the
Q188: Calculate the hydronium ion concentration in an
Q189: Which Br∅nsted-Lowry acid has the strongest conjugate
Q190: What is the conjugate acid of the