Multiple Choice
What is the hydronium ion concentration in a solution prepared by mixing 50.00 mL of 0.10 M HCN with 50.00 mL of 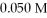 NaCN? Assume that the volumes of the solutions are additive and that Ka = 4.9 × 10-10 for HCN.
NaCN? Assume that the volumes of the solutions are additive and that Ka = 4.9 × 10-10 for HCN.
A) 2.4 × 10-10 M
B) 4.9 × 10-10 M
C) 9.8 × 10-10 M
D) 7.0 × 10-6 M
Correct Answer:

Verified
Correct Answer:
Verified
Q184: The artist's pigment cadmium yellow,CdS,has a water
Q185: Which of the following titrations result in
Q186: What is the hydronium ion concentration in
Q187: Which of these neutralization reactions has a
Q188: The following pictures represent solutions at various
Q190: The neutralization constant K<sub>n</sub> for the neutralization
Q191: What is the pH at the equivalence
Q192: Identify the following solutions as acidic,basic,or inert.<br>NaNO<sub>2</sub>
Q193: The following plot shows a titration curve
Q194: What is not a correct expression for