Multiple Choice
Which metal sulfides can be precipitated from a solution that is 0.01 M in Mn2+,Zn2+,Pb2+ and Cu2+ and 0.10 M in H2S at a pH of 1.0? 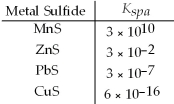
A) MnS
B) CuS
C) PbS,CuS
D) ZnS,PbS,CuS
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q87: What is the least soluble salt of
Q88: What is the molar solubility of lead(II)chromate
Q89: The solution formed upon adding 80.00 mL
Q90: What is the pH of the resulting
Q91: What is the pH of a solution
Q93: What volume of 0.100 M NaOH is
Q94: What is the pH of the resulting
Q95: What is the Henderson-Hasselbalch equation for the
Q96: The neutralization constant K<sub>n</sub> for the neutralization
Q97: The dissociation equilibrium constants for the protonated