Multiple Choice
Which metal sulfides can be precipitated from a solution that is 0.01 M in Mn2+,Zn2+,Pb2+ and Cu2+ and 0.10 M in H2S at a pH of 0.50? 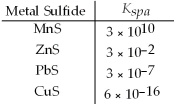
A) MnS
B) CuS
C) PbS,CuS
D) ZnS,PbS,CuS
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q8: The following pictures represent solutions that contain
Q111: Which of the following reactions are not
Q112: The following pictures represent solutions at various
Q113: Calculate the K<sub>sp</sub> for silver sulfite if
Q114: The addition of _ mL of 0.3000
Q116: What is the pH of a buffer
Q117: The following pictures represent solutions at various
Q118: Which of the following combinations of chemicals
Q119: Use the graphs below to answer the
Q120: Calculate the solubility (in g/L)of silver carbonate