Multiple Choice
The following pictures represent solutions that contain a weak acid HA and/or its potassium salt KA.Unshaded spheres represent H atoms and shaded spheres represent A- ions.(K+,H3O+,OH-,and solvent H2O molecules have been omitted for clarity. ) 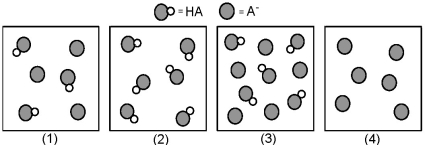
-Which solution has the largest percent dissociation of HA?
A) (1)
B) (2)
C) (3)
D) (4)
Correct Answer:

Verified
Correct Answer:
Verified
Q117: The following pictures represent solutions at various
Q118: Which of the following combinations of chemicals
Q119: Use the graphs below to answer the
Q120: Calculate the solubility (in g/L)of silver carbonate
Q121: The following plot shows a titration curve
Q123: 0.10 M potassium chromate is slowly added
Q124: What is the molar solubility of AgCl
Q125: Calculate the molar solubility of thallium(I)chloride in
Q126: What is the silver ion concentration for
Q127: The following pictures represent solutions of AgCl,which