Multiple Choice
The following pictures represent solutions that contain a weak acid HA (pKa = 5.0) and its potassium salt KA.Unshaded spheres represent H atoms and shaded spheres represent A- ions.(K+,H3O+,OH-,and solvent H2O molecules have been omitted for clarity. ) 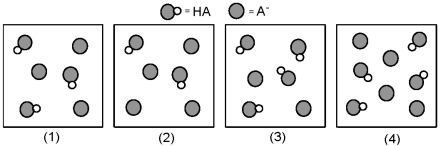
-For which of these solutions is pH = pKa?
A) All have pH = pKa.
B) (1) , (2) and (3)
C) (1) and (4)
D) (2) and (3)
Correct Answer:

Verified
Correct Answer:
Verified
Q40: When 110 mL of 0.12 M NaF
Q41: In which of the following solutions would
Q42: Formic acid (HCO<sub>2</sub>H,K<sub>a</sub> = 1.8 × 10<sup>-4</sup>)is
Q43: When equal molar amounts of the following
Q44: Precipitation of an ionic compound will occur
Q46: TRIS {(HOCH<sub>2</sub>)<sub>3</sub>CNH<sub>2</sub>} is one of the most
Q47: What is the pH of a solution
Q48: In which of the following solutions would
Q49: Which is a net ionic equation for
Q50: Use the graphs below to answer the