Multiple Choice
The following pictures represent solutions at various stages in the titration of a weak diprotic acid H2A with aqueous KOH.Unshaded spheres represent H atoms,black spheres represent oxygen atoms,and shaded spheres represent A2- ions.(K+,H3O+ initially present,OH- initially present and solvent water molecules have been omitted for clarity) . 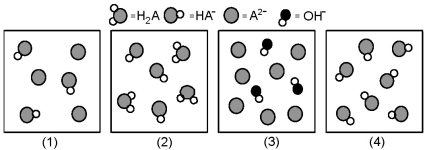
-Which picture represents the system halfway between the first and second equivalence points?
A) (1)
B) (2)
C) (3)
D) (4)
Correct Answer:

Verified
Correct Answer:
Verified
Q191: What is the pH at the equivalence
Q192: Identify the following solutions as acidic,basic,or inert.<br>NaNO<sub>2</sub>
Q193: The following plot shows a titration curve
Q194: What is not a correct expression for
Q195: A buffer prepared by mixing equal moles
Q196: The dissociation equilibrium constants for the protonated
Q197: What is the molar solubility of AgCl
Q199: What is the pH of a solution
Q200: The following pictures represent solutions of CaCO<sub>3</sub>,which
Q201: Which is a net ionic equation for