Multiple Choice
The following pictures represent solutions of AgCl,which may also contain ions other than Ag+ and Cl- which are not shown.Gray spheres represent Ag+ ions and dotted spheres represent Cl- ions. 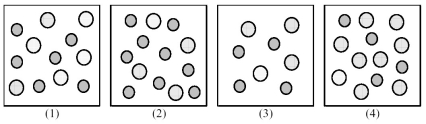
-If solution (1) is a saturated solution of AgCl,which of solutions (1) -(4) represents the solution after a small amount of HNO3 is added and equilibrium is restored?
A) (1)
B) (2)
C) (3)
D) (4)
Correct Answer:

Verified
Correct Answer:
Verified
Q127: The following pictures represent solutions of AgCl,which
Q128: What is the pH of a solution
Q129: The following pictures represent solutions of CaCO<sub>3</sub>,which
Q130: Which of the following buffer solutions will
Q131: Sodium hypochlorite,NaOCl,is the active ingredient in household
Q133: The neutralization constant K<sub>n</sub> for the neutralization
Q134: What is the approximate value of the
Q135: The following pictures represent solutions of CaCO<sub>3</sub>,which
Q136: The following pictures represent solutions of CuS,which
Q137: Barium hydroxide is slightly soluble in water,with