Multiple Choice
Given 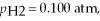
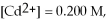 and
and 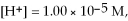 calculate E at 25°C for a cell based on the reaction:
calculate E at 25°C for a cell based on the reaction: 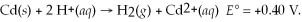
A) -0.09 V
B) +0.12 V
C) +0.15 V
D) +0.30 V
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q21: Based on the balanced chemical equation shown
Q22: A galvanic cell consists of one half-cell
Q23: In the shorthand notation for a galvanic
Q24: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -The cell reaction
Q25: What is the shorthand notation that represents
Q27: Identify the anode and cathode half-reactions and
Q28: According to the balanced equation shown below,1.00
Q29: The standard cell potential for the following
Q30: For the reaction 2 Al(s)+ 3 Co<sup>2+</sup>(aq)→
Q42: How many grams of nickel metal are