Multiple Choice
Consider the galvanic cell shown below. 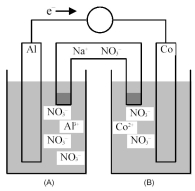
-Identify the anode and cathode,and indicate the direction of ion flow to and from each electrode.
A) Al is the anode and Co is the cathode;Al3+ ions flow to the anode and Co2+ ions flow from the cathode.
B) Al is the anode and Co is the cathode;Co2+ ions flow to the cathode and Al3+ ions flow from the anode.
C) Co is the anode and Al is the cathode;Al3+ ions flow to the cathode and Co2+ ions flow from the anode.
D) Co is the anode and Al is the cathode;Co2+ ions flow to the anode and Al3+ ions flow from the cathode.
Correct Answer:

Verified
Correct Answer:
Verified
Q1: Which is not true for standard electrode
Q2: For the hypothetical reaction A + B<sup>x</sup>
Q4: Given the half-cell potentials below,calculate the cell
Q5: Galvanized steel is steel coated with a
Q6: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -According to Table
Q7: Calculate the cell potential E at 25°C
Q8: How long must a constant current of
Q9: For the galvanic cell reaction,expressed below using
Q10: For a galvanic cell,the cathode has a
Q11: According to the balanced equation shown below,3.00