Multiple Choice
Consider the galvanic cell shown below. 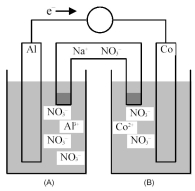
-What is the shorthand notation for the cell?
A) Al(s) | Al3+(aq) || Co(s) | Co2+(aq)
B) Al(s) | Al3+(aq) || Co2+(aq) | Co(s)
C) Co(s) | Co2+(aq) || Al(s) | Al3+(aq)
D) Co(s) | Co2+(aq) || Al3+(aq) | Al(s)
Correct Answer:

Verified
Correct Answer:
Verified
Q25: What is the shorthand notation that represents
Q59: An electrolytic cell is<br>A)a battery.<br>B)a cell in
Q60: How many grams of chromium metal are
Q61: What is the reduction half-reaction for the
Q62: The standard potential for the following galvanic
Q63: Consider the following table of standard reduction
Q66: What is the oxidation half reaction in
Q67: What are the coefficients in front of
Q68: O<sub>2</sub>(g)+ 4 H<sup>+</sup>(aq)+ 4 e<sup>-</sup> → 2
Q69: A galvanic cell consists of one half-cell