Multiple Choice
Shown below is a galvanic cell with anode compartment b containing anode a and cathode compartment d containing cathode c.Electrons flow through wire f,ions flow through salt bridge e,and the cell potential is read using voltmeter g.
This galvanic cell uses the reaction: 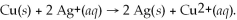
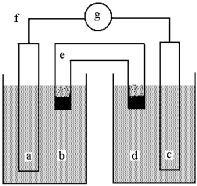
-Identify and give the sign of each electrode.
A) a is Ag and (+) ,c is Cu and (-) .
B) a is Ag and (-) ,c is Cu and (+) .
C) a is Cu and (+) ,c is Ag and (-) .
D) a is Cu and (-) ,c is Ag and (+) .
Correct Answer:

Verified
Correct Answer:
Verified
Q88: A galvanic cell consists of a Al<sup>3+</sup>/Al
Q89: Which of the following reactions is most
Q90: In the galvanic cell represented by the
Q91: Based on the half-reactions and their respective
Q92: Which requires the most electricity (in terms
Q94: During an electrochemical reaction,electrons move through the
Q95: If the cell reaction involves ions in
Q96: What are the coefficients in front of
Q97: Based on the balanced chemical equation shown
Q98: Redox reactions occurring in acid are evident