Multiple Choice
The initial concentrations of Ag+(aq) and Cu2+(aq) are both 1.0 M.What will happen to the cell voltage if 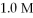 Cu(NO3) 2 is added to the compartment containing the 1.0 M Cu2+(aq) ? The cell voltage will
Cu(NO3) 2 is added to the compartment containing the 1.0 M Cu2+(aq) ? The cell voltage will
A) decrease.
B) increase.
C) remain the same.
D) can't tell from the information given
Correct Answer:

Verified
Correct Answer:
Verified
Q140: The gas OF<sub>2</sub> can be produced from
Q176: Based on the half-reactions and their respective
Q178: In a galvanic cell,the half-reaction MnO<sub>4</sub><sup>-</sup>(aq)+ 8
Q179: Determine the number of water molecules necessary
Q180: What is the value of the equilibrium
Q182: Which statement below is not true?<br>A)The cell
Q183: Which cell involves a nonspontaneous redox reaction?<br>A)concentration
Q184: What species is oxidized in the reaction:
Q185: What is the balanced chemical equation for
Q186: What is the reduction half-reaction for the