Multiple Choice
Consider the galvanic cell shown below. 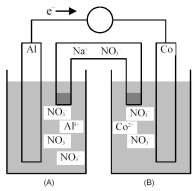
-What is the quantitative change in the cell voltage on increasing the ion concentration in the cathode compartment by a factor of 10?
A) +0.03 V
B) +0.02 V
C) -0.02 V
D) -0.03 V
Correct Answer:

Verified
Correct Answer:
Verified
Q154: A salt bridge is used to<br>A)provide reactants
Q155: Calculate the value of the reaction quotient,Q,for
Q156: In the unbalanced equation shown below how
Q157: What are the coefficients in front of
Q158: Based on the balanced chemical equation shown
Q160: Which of the following statements concerning the
Q161: Given: Ag<sup>+</sup>(aq)+ e<sup>-</sup> → Ag(s)E° = +0.799
Q162: Which is most often used in the
Q163: For a galvanic cell that uses the
Q164: Consider the half-reaction: MnO<sub>4</sub><sup>-</sup>(aq)+ 8 H<sup>+</sup>(aq)+ 5