Multiple Choice
Given that Br2(g) + 2 e- → 2 Br-(aq) is the reduction half-reaction for the overall reaction 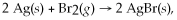 what is the oxidation half reaction?
what is the oxidation half reaction?
A) Ag(s) → Ag+(aq) + e-
B) Ag(s) + Br-(aq) → AgBr(s) + e-
C) Ag(s) + Br2(g) + e- → AgBr(s) + Br-(aq)
D) 2 Br-(aq) → Br2(g) + 2 e-
Correct Answer:

Verified
Correct Answer:
Verified
Q28: According to the balanced equation shown below,1.00
Q29: The standard cell potential for the following
Q30: For the reaction 2 Al(s)+ 3 Co<sup>2+</sup>(aq)→
Q32: Based on the balanced chemical equation shown
Q34: Doubling all the coefficients in the equation
Q35: For a particular cell based on the
Q36: Shown below are the reactions occurring in
Q37: The nickel-cadmium battery cell has a standard
Q38: Calculate the value of the reaction quotient,Q,for
Q42: How many grams of nickel metal are