Multiple Choice
A shorthand way of writing transmutation equations is to show the bombarding particle and the subsequent emitted particle in parentheses.Which particle is produced by 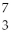 Li (p,α) ?
Li (p,α) ?
A) 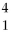
H
B) 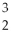
He
C) 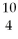
Be
D) 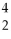
He
Correct Answer:

Verified
Correct Answer:
Verified
Q53: In addition to a beta particle,what is
Q54: The mass defect for the chemical reaction
Q55: An alpha particle is<br>A) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt="An
Q56: An experiment with <sup>55</sup>Co takes 47.5 hours.At
Q57: The nuclear decay process that involves the
Q59: Breaking a chemical bond requires approximately 10<sup>2</sup>
Q60: Which ionizing radiation has the greatest energy?<br>A)α<br>B)β<br>C)γ<br>D)χ
Q61: What type of shielding can be used
Q62: What is the mass change in grams
Q63: What percentage of a radioactive substance remains