Multiple Choice
The first transition series metals are shown below. 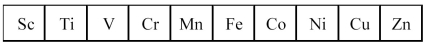
-Which has the most positive standard oxidation potential?
A) Sc
B) V
C) Cu
D) Zn
Correct Answer:

Verified
Correct Answer:
Verified
Q6: What chemical equation represents the best method
Q8: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -Which element indicated
Q9: What is a representative orbital-filling diagram for
Q10: The second transition series metals are shown
Q12: What is the crystal field energy level
Q13: What is the ground-state electron configuration for
Q14: What is the coordination number of the
Q15: Consider the following isomers of [Co(NH<sub>3</sub>)<sub>4</sub>Br<sub>2</sub>]<sup>+</sup>.The black
Q16: Describe what happens when 3.0 M NH<sub>3</sub>
Q100: What is the ground-state electron configuration for