Multiple Choice
The second transition series metals are shown below. 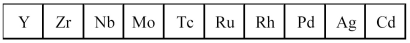
-Which has the highest melting point?
A) Y
B) Mo
C) Tc
D) Cd
Correct Answer:

Verified
Correct Answer:
Verified
Q89: A chromium(III)ion forms a complex ion with
Q90: Which of the following elements has the
Q91: Though we would expect an increase in
Q92: The complex [Ni(CN)<sub>4</sub>]<sup>2-</sup> is diamagnetic and the
Q93: The coordination number of cobalt in [Co(NH<sub>3</sub>)<sub>4</sub>Cl<sub>2</sub>]F
Q95: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -Which is the
Q96: Consider the following isomers of [Co(NH<sub>3</sub>)<sub>4</sub>Br<sub>2</sub>]<sup>+</sup>.The black
Q97: Which transition element has the highest melting
Q98: The first transition series metals are shown
Q99: Which Pt(II)complexes are geometric isomers of each