Multiple Choice
The third transition series metals are shown below. 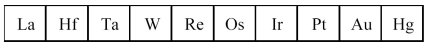
-Which has the largest atomic radius?
A) La
B) W
C) Os
D) Hg
Correct Answer:

Verified
Correct Answer:
Verified
Q153: What is the name of the complex
Q154: Which complex cannot exist as enantiomers?<br>A)[CoF<sub>2</sub>(H<sub>2</sub>O)<sub>4</sub>]<sup>+</sup><br>B)[Co(gly)<sub>3</sub>]<sup>3+</sup><br>C)cis-[CrCl<sub>2</sub>(C<sub>2</sub>O<sub>4</sub>)<sub>2</sub>]<sup>+</sup><br>D)[Fe(C<sub>2</sub>O<sub>4</sub>)<sub>2</sub>(en)]<sup>-</sup>
Q155: Based on the variation in Z<sub>eff</sub>,which oxoanion
Q156: What are two of the major components
Q157: Which first row transition element has the
Q159: What is the oxidation state of the
Q160: Which of the following species is diamagnetic?<br>A)an
Q161: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -Which element indicated
Q162: Platinum uses _ hybrid orbitals to accept
Q163: How many electrons are in the ground