Multiple Choice
The third transition series metals are shown below. 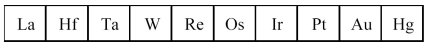
-Which has the smallest atomic radius?
A) La
B) W
C) Os
D) Hg
Correct Answer:

Verified
Correct Answer:
Verified
Q122: What is the ground-state electron configuration for
Q123: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -Which group of
Q124: The third transition series metals are shown
Q125: Using shorthand notation,the electron configuration of Co<sup>3+</sup>
Q126: The absorbance spectrum of a complex along
Q128: Which of the following complex ions is
Q129: Which is a complex ion?<br>A)FeBr<sub>3</sub><br>B)CrO<sub>4</sub><sup>2-</sup><br>C)[Cr(H<sub>2</sub>O)<sub>6</sub>]<sup>3+</sup><br>D)Cr<sub>2</sub>O<sub>7</sub><sup>2-</sup>
Q130: How many unpaired electrons will Co have
Q131: Which of the following can function as
Q132: Transition series elements are all<br>A)gases.<br>B)metals.<br>C)nonmetals.<br>D)semimetals.