Multiple Choice
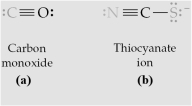
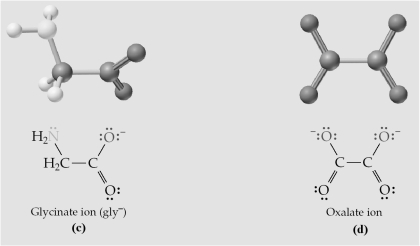
-Which of the above are bidentate ligands?
A) All are bidentate.
B) (a) and (b)
C) (c) and (d)
D) None are bidentate.
Correct Answer:

Verified
Correct Answer:
Verified
Q30: For transition elements,which of the following occurs
Q31: The second transition series metals are shown
Q32: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -Which
Q33: Which of the following chromium species is
Q34: What is the highest possible oxidation state
Q36: Write the chemical formula for aquabromobis(ethylenediamine)chromium(III)chloride.<br>A)[CrBr(H<sub>2</sub>O)(en)]Cl<br>B)[CrBr<sub>2</sub>(H<sub>2</sub>O)(en)]Cl<sub>2</sub><br>C)[CrBr(H<sub>2</sub>O)(en)<sub>2</sub>]Cl<sub>2</sub><br>D)[CrBr(H<sub>2</sub>O)(en)<sub>2</sub>]Cl<sub>3</sub>
Q37: The color exhibited by coordination compounds is
Q38: The number of unpaired electrons in Co(NH<sub>3</sub>)<sub>6</sub><sup>3+</sup>
Q39: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -Using the above
Q40: What is the characteristic outer electron configuration