Multiple Choice
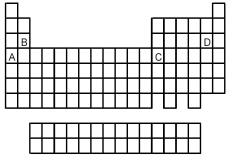
-Which of the elements indicated on the above periodic table has the highest ionization energy?
A) element A
B) element B
C) element C
D) element D
Correct Answer:

Verified
Correct Answer:
Verified
Q193: At 25°C the elements indicated by the
Q194: The elements indicated by the shaded area
Q195: Which alkali metal forms preferentially an oxide
Q196: Which of the hydrogen halide acids is
Q197: Ionization energy of main-group elements generally<br>A)increases from
Q199: Which one of the following is not
Q200: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -Which of the
Q201: In the following pictures representing binary hydrides
Q202: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -What is the
Q203: The following pictures represent binary hydrides,AH<sub>x</sub>,where A