Multiple Choice
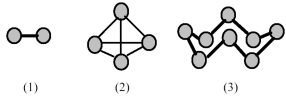
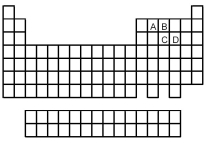
-Which of the elements indicated on the above periodic table has a common allotrope stable at 25°C having molecular structure (1) ?
A) element A
B) element B
C) element C
D) element D
Correct Answer:

Verified
Correct Answer:
Verified
Q37: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -Which element shown
Q38: Predict the product(s)of the reaction of Br<sub>2</sub>(aq)with
Q39: The most acidic oxides of the group
Q40: An incorrect statement about the alkaline earth
Q41: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -Which
Q43: Which elements of group 3A are commonly
Q44: Carbon combines with some metals and semimetals
Q45: In the picture representing binary hydride AH<sub>x</sub>,lightly-shaded
Q46: The number of semimetals in group 4A
Q47: What is not a reaction commonly associated