Multiple Choice
The following pictures represent binary hydrides,AHx,where A = Na,Ti,C,or Cl.Lightly-shaded spheres represent H atoms or ions and darkly-colored spheres represent atoms or ions of element A. 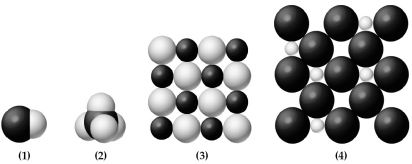
-Which hydride dissolves in water to form an acidic solution?
A) hydride (1)
B) hydride (2)
C) hydride (3)
D) hydride (4)
Correct Answer:

Verified
Correct Answer:
Verified
Q54: Which is not generally considered to be
Q55: What is the ground state valence shell
Q56: What statement is inconsistent about carbon monoxide?<br>A)It
Q57: Of Na<sub>2</sub>O,SO<sub>2</sub>,and SO<sub>3</sub>,equimolar aqueous solutions of _
Q58: The net ionic reaction of solid CaH<sub>2</sub>
Q60: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -Which element shown
Q61: Diopside,CaMgSi<sub>2</sub>O<sub>6</sub>,is an example of a(n)_ (orthosilicate,single-strand silicate,double-strand
Q62: What is the most stable oxidation state
Q63: In which compound is nitrogen in its
Q64: Covalent hydrides of the type MH<sub>3</sub> are