Multiple Choice
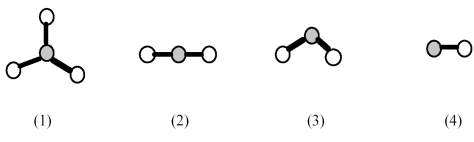
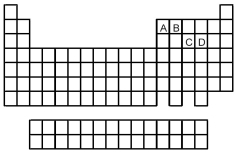
-Which element indicated on the above periodic table forms a binary oxide with molecular structure (1) shown above?
A) element A
B) element B
C) element C
D) element D
Correct Answer:

Verified
Correct Answer:
Verified
Q163: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -Which
Q164: The elements indicated by the shaded area
Q165: The charge,n,on the cyclic anion Si<sub>6</sub>O<sub>18</sub><sup>n</sup> that
Q166: In the following pictures representing binary hydrides
Q167: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -Using the above
Q169: Which statement is most inconsistent with the
Q170: What is the structure of white phosphorus?<br>A)cage
Q171: Group 2A metals tend to be somewhat
Q172: What is not an appropriate method for
Q173: When the equation for the reaction of