Short Answer
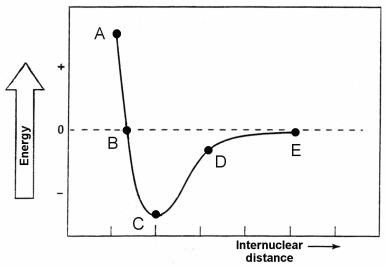
-Which point identifies the bond length between the two atoms of the diatomic molecule whose potential energy is shown on the graph?
Correct Answer:

Verified
Correct Answer:
Verified
Q36: Although noble gases do not normally form
Q40: The formula for carbon disulfide is<br>A)CS<sub>2.</sub><br>B)C<sub>2</sub>S.<br>C)CSi<sub>2.</sub><br>D)CaS<sub>2.</sub><br>E)(CS)<sub>2.</sub>
Q49: The element in the list given that
Q50: In a covalent compound the bond length
Q52: Match the following.<br>-carbon tetrachloride<br>A)OF<sub>2</sub><br>B)BrF<sub>5</sub><br>C)CCI<sub>4</sub><br>D)N<sub>2</sub>O<sub>4</sub><br>E)SF<sub>6</sub><br>F)CO<br>G)ICl<br>H)SO<sub>2</sub><br>I)CH<sub>4</sub><br>J)IF<sub>7</sub><br>K)PCI<sub>3</sub><br>L)NH<sub>3</sub>
Q54: For the structure shown,the most likely elements
Q55: A chemical bond formed between two identical
Q58: Match the following.<br>-carbon monoxide<br>A)OF<sub>2</sub><br>B)BrF<sub>5</sub><br>C)CCI<sub>4</sub><br>D)N<sub>2</sub>O<sub>4</sub><br>E)SF<sub>6</sub><br>F)CO<br>G)ICl<br>H)SO<sub>2</sub><br>I)CH<sub>4</sub><br>J)IF<sub>7</sub><br>K)PCI<sub>3</sub><br>L)NH<sub>3</sub>
Q65: The molecule SiCl<sub>4</sub> has a _ shape.<br>A)bent<br>B)linear<br>C)planar<br>D)pyramidal<br>E)tetrahedral
Q69: When a non-metal atom bonds with another