Multiple Choice
A section of the Periodic Table containing main group elements is shown.Which bond would be least polar? 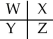
A) WX
B) YZ
C) WZ
D) WY
E) cannot be determined without more specific information
Correct Answer:

Verified
Correct Answer:
Verified
Q4: Which representation of a hydrogen molecule is
Q13: A chemical bond formed when two atoms
Q14: Consider the molecule SiCl<sub>4</sub>.The electronegativity values for
Q26: The formula for sulfur hexabromide is<br>A)SF<sub>6.</sub><br>B)SBr<sub>6.</sub><br>C)SBr<sub>4.</sub><br>D)SiBr<sub>6.</sub><br>E)SiB<sub>6.</sub>
Q53: Draw the Lewis structure for CCl<sub>4</sub>.What is
Q57: The covalent bonding model is most useful
Q58: Which element listed is the least electronegative?<br>A)nitrogen<br>B)hydrogen<br>C)oxygen<br>D)fluorine<br>E)chlorine
Q60: Covalent compounds form distinct molecules,and therefore may
Q62: Match the following.<br>-phosphorus trichloride<br>A)OF<sub>2</sub><br>B)BrF<sub>5</sub><br>C)CCI<sub>4</sub><br>D)N<sub>2</sub>O<sub>4</sub><br>E)SF<sub>6</sub><br>F)CO<br>G)ICl<br>H)SO<sub>2</sub><br>I)CH<sub>4</sub><br>J)IF<sub>7</sub><br>K)PCI<sub>3</sub><br>L)NH<sub>3</sub>
Q70: The compound BrF<sub>3</sub> would be called<br>A)boron trifluoride.<br>B)triboron