Multiple Choice
Which is not a correct statement of Charles's Law?
A) For a gas sample at constant pressure,temperature and volume are inversely proportional.
B) volume α temperature
C) V/T = a constant
D) 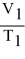
=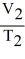
E) none of the above
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q7: What volume will be occupied by 8.00
Q9: A solid compound which has no definite
Q27: What is the pressure in a 1.00
Q35: Which description best fits a solid?<br>A)definite shape
Q49: List and describe two unique properties of
Q51: As the temperature of a liquid is
Q55: Sketch a graph illustrating Boyle's Law.Use a
Q67: The amount of energy involved in melting
Q68: Most gases show variations from ideality at
Q78: Which measurement represents the largest value of