Essay
Consider the reaction: H2SO3 + HCO3- 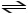
H2CO3 + HSO3-
a)Identify the acid,base,conjugate acid and conjugate base
b)Identify two substances from the reaction which could be used to prepare a buffer.
Correct Answer:

Verified
a)Acid: H2SO3; Conjugate Base: H...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q6: Which of the following is a triprotic
Q23: An increase in solution pH corresponds to<br>A)an
Q25: If the concentration of H<sub>3</sub>O<sup>+</sup> in an
Q29: Which one of the following is the
Q43: Which of these,if dissolved in 1.0 L
Q76: What is the [H<sub>3</sub>O<sup>+</sup>] in a solution
Q77: Match the following.<br>-Bronsted-Lowry theory<br>A)an acid-base theory that
Q79: What is the pH of a solution
Q84: Ammonia reacts with acids because<br>A)it contains the
Q99: If the concentration of H<sub>3</sub>O<sup>+</sup> is 3.5