Multiple Choice
Consider the isotope 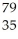
Br.The atomic number is ________,and the mass number is ________.
A) 79;35
B) 35;79
C) 44;35
D) 35;44
E) 35;114
Correct Answer:

Verified
Correct Answer:
Verified
Q55: How many electrons can occupy the 3d
Q56: What is the electron configuration of S?<br>A)1s<sup>2
Q57: The element with the electron configuration<br>1s<sup>2 </sup>2s<sup>2
Q58: Which group contains only noble gases?<br>A)Ni,Pd,Pt<br>B)Si,Ge,As<br>C)Ce,Pr,Nd<br>D)Kr,Xe,Rn<br>E)Po,Fr,Ac
Q59: The value of A for an atom
Q61: Which group of elements contains only non-metals?<br>A)Mg,Ca,Sr<br>B)V,Cr,Mn<br>C)Cl,Ar,K<br>D)P,As,Se<br>E)C,S,I
Q62: What is the maximum number of electrons
Q63: Magnesium is an example of a(an)<br>A)alkali metal.<br>B)alkaline
Q64: The element with the electron configuration 1s<sup>2
Q65: The maximum number of electrons in any