Multiple Choice
Which of the following represents a pair of isotopes?
A) 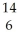 C,
C,  N
N
B) 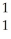 H,
H, 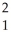 H
H
C) 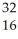 S,
S,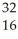 S2
S2
D) O2,O3
E) all are pairs of isotopes
Correct Answer:

Verified
Correct Answer:
Verified
Q86: The number of neutrons in an atom
Q87: How many neutrons does an atom of
Q88: What fourth period element is represented by
Q89: Valence electrons in the main group elements
Q90: Which group contains only d-block elements?<br>A)Ni,Pd,Pt<br>B)Si,Ge,As<br>C)Ce,Pr,Nd<br>D)Kr,Xe,Rn<br>E)Po,Fr,Ac
Q91: The number of valence electrons in a
Q92: Cobalt is element 27.Cobalt-60 is used in
Q93: Which group contains only f-block elements?<br>A)Ni,Pd,Pt<br>B)Si,Ge,As<br>C)Ce,Pr,Nd<br>D)Kr,Xe,Rn<br>E)Po,Fr,Ac
Q94: Which group contains only p-block elements?<br>A)N,S,Br<br>B)Mn,Cu,Ag<br>C)K,Mg,Al<br>D)Ce,Pr,Nd<br>E)Na,P,Cl
Q96: The number of valence electrons in an