Multiple Choice
Match the following.
-calcium sulfate
A) 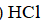
B) 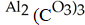
C) 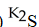
D) 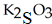
E) 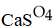
F) 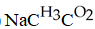
G) 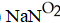
H) 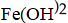
I) 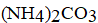
J) 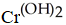
K) 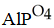
L) 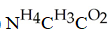
Correct Answer:

Verified
Correct Answer:
Verified
Q8: What is the most likely charge on
Q9: When hydrochloric acid dissolves in water what
Q10: The name of the compound with formula
Q11: The HCO<sub>3</sub><sup>1-</sup>ion is called<br>A)hydrogen carbonate.<br>B)hydrogen carbide.<br>C)carbide.<br>D)carbonate.<br>E)carbonite.
Q12: The formula for ammonium hydroxide is<br>A)OHNH<sub>4.</sub><br>B)NH<sub>4</sub>NO<sub>3.</sub><br>C)NH<sub>4</sub>O.<br>D)NH<sub>4</sub>OH.<br>E)Al(OH)<sub>3.</sub>
Q14: The permanganate ion is composed of<br>A)one atom
Q15: Which pair of elements is most likely
Q16: The charge on a sulfide ion is<br>A)3+.<br>B)2+.<br>C)0.<br>D)2-.<br>E)3-.
Q17: What is the name of AlCl<sub>3</sub>?<br>A)aluminum chloride<br>B)aluminum(III)chloride<br>C)aluminum
Q18: A small negatively charged particle formed when