Multiple Choice
For the dot structure shown the most likely elements are X =________ and Y = ________. 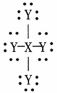
A) carbon;hydrogen
B) carbon;fluorine
C) carbon;oxygen
D) hydrogen;carbon
E) fluorine;carbon
Correct Answer:

Verified
Correct Answer:
Verified
Q34: The bond angle in the molecule H<sub>2</sub>S
Q35: Which group in the Periodic Table is
Q36: Although noble gases do not normally form
Q37: In a Lewis dot structure the electrons
Q38: What is the molecular geometry of PH<sub>3</sub>?<br>A)linear<br>B)trigonal
Q40: The formula for carbon disulfide is<br>A)CS<sub>2.</sub><br>B)C<sub>2</sub>S.<br>C)CSi<sub>2.</sub><br>D)CaS<sub>2.</sub><br>E)(CS)<sub>2.</sub>
Q41: The VSEPR model of molecular structure requires
Q42: A section of the Periodic Table containing
Q43: A molecule in which the central atom
Q44: Match the following.<br>-carbon monoxide<br>A)ICl<br>B)C <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4943/.jpg" alt="Match