Multiple Choice
A section of the Periodic Table containing main group elements is shown.If the elements W,X,Y,and Z have electronegativity values of 1.0,2.0,2.5,and 3.5,respectively,which bond is ionic? 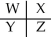
A) WY
B) WZ
C) XY
D) XZ
E) YZ
Correct Answer:

Verified
Correct Answer:
Verified
Q58: Which element listed is the least electronegative?<br>A)nitrogen<br>B)hydrogen<br>C)oxygen<br>D)fluorine<br>E)chlorine
Q59: Explain why it is unlikely that an
Q60: Covalent compounds form distinct molecules,and therefore may
Q61: Which element is most likely to form
Q62: Which set of properties would identify an
Q64: Draw the Lewis structure for the a
Q65: The molecule SiCl<sub>4</sub> has a _ shape.<br>A)bent<br>B)linear<br>C)planar<br>D)pyramidal<br>E)tetrahedral
Q66: Consider a bent molecule,such as H<sub>2</sub>Se,in which
Q67: Match the following.<br>-carbon tetrachloride<br>A)ICl<br>B)C <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4943/.jpg" alt="Match
Q68: Which group contains only elements which normally