Multiple Choice
Consider the reaction shown and identify the statement that is not true.
CaCO3(s) 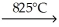 CaO(s) + CO2(g)
CaO(s) + CO2(g)
A) This reaction is balanced as written.
B) The reactant must be heated for this reaction to occur.
C) The products are a solid and a gas.
D) Water must be present for this reaction to occur.
E) There are no solutions used in this reaction.
Correct Answer:

Verified
Correct Answer:
Verified
Q51: When the reaction shown is correctly balanced,the
Q52: Reduction is the process of<br>A)gaining hydrogen.<br>B)losing oxygen.<br>C)gaining
Q53: Which reaction is not an example of
Q54: As a pure element the oxidation number
Q55: When a substance loses electrons it is
Q57: Which statement regarding balanced chemical equations is
Q58: In which of the following is the
Q59: What are the spectator ions in the
Q60: When a solution of iron(III)nitrate is mixed
Q61: Which reaction is an example of both