Multiple Choice
Match the following.
-M(s) + 2 LX(aq) → 2 L(s) + M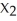 (aq)
(aq)
A) redox
B) acid-base
C) precipitation
Correct Answer:

Verified
Correct Answer:
Verified
Q42: The reaction 2 AgNO<sub>3</sub>(aq)+ K<sub>2</sub>SO<sub>4</sub>(aq)→ 2 KNO<sub>3</sub>(aq)+
Q43: 2 AgNO<sub>3</sub>(aq) + K<sub>2</sub>SO<sub>4</sub> (aq) → 2
Q44: In a typical oxidation-reduction reaction the electrons
Q45: 2 AgNO<sub>3</sub>(aq)+ K<sub>2</sub>SO<sub>4</sub>(aq)→ 2 KNO<sub>3</sub>(aq)+ Ag<sub>2</sub>SO<sub>4</sub>(s)<br>The spectator
Q46: When the reaction shown is correctly balanced,the
Q48: The formation of which one of the
Q49: In a precipitation reaction the insoluble product
Q50: The combination of ions least likely to
Q51: When the reaction shown is correctly balanced,the
Q52: Reduction is the process of<br>A)gaining hydrogen.<br>B)losing oxygen.<br>C)gaining