Multiple Choice
The molecule shown is a ________ alcohol because ________. 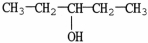
A) primary;it has one -OH group
B) primary;its -OH group is on the end of the molecule
C) secondary;the carbon bonded to the -OH group is bonded to two other carbons
D) secondary;each group bonded to the hydroxyl carbon contains two carbon atoms
E) tertiary;the -OH is bonded to the number 3 carbon
Correct Answer:

Verified
Correct Answer:
Verified
Q1: The major product resulting from the dehydration
Q2: Which molecule shown is a primary alcohol?<br>A)<br><img
Q3: What is the IUPAC name of the
Q4: What is the inorganic compound that can
Q6: Match the following.<br>-disulfide<br>A)<img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4943/.jpg" alt="Match the following.
Q7: Treatment of CH<sub>3</sub>CH<sub>2</sub>OH with an excess amount
Q8: An alcohol is classified as primary,secondary or
Q9: The IUPAC name of the alcohol shown
Q10: Which functional group will cause a compound
Q11: Which alcohol should be used to produce