Multiple Choice
The same activity is measured for two different isotope samples. One sample contains 0.0450 kg of 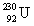 (atomic mass = 230.033 937 u, t1/2 = 20.8 days) . The second sample contains an unknown amount of
(atomic mass = 230.033 937 u, t1/2 = 20.8 days) . The second sample contains an unknown amount of 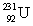 (atomic mass = 231.036 264 u, t1/2 = 4.3 days) . What is the mass of the second sample?
(atomic mass = 231.036 264 u, t1/2 = 4.3 days) . What is the mass of the second sample?
A) 0.0093 kg
B) 0.23 kg
C) 0.110 kg
D) 0.037 kg
E) 0.0450 kg
Correct Answer:

Verified
Correct Answer:
Verified
Q14: Which one of the following statements
Q15: What is the wavelength of the
Q16: Radiation or(and) particles emerge(s) from a
Q17: An isotope of krypton has a half-life
Q18: Complete the following sentence: In a
Q20: Osmium has atomic number 76. A particular
Q21: Which one of the following thicknesses
Q22: Which one of the following quantities is
Q23: How many neutrons are there in the
Q24: Determine the activity of 6.0 × 10<sup>12</sup>